- HOME
- > NEWS / EVENTS
- > 2025
- > Release of High-Resolution PET System PositView Utilizing High Resolution to Support Research and Development into Diagnosis and Early Treatment for Dementia
NEWS
July 25, 2025
Release of High-Resolution PET System PositView Utilizing High Resolution to Support Research and Development into Diagnosis and Early Treatment for Dementia
Shimadzu Corporation has obtained premarketing notification from the U.S. Food and Drug Administration (FDA) for its PositView PET system that is specialized for head and breast examinations and starts marketing in the US. Positron emission tomography (PET) is a non-invasive diagnostic imaging method by administering drugs into the body that accumulate at specific targets.
PET could be used for obtaining images of the accumulation of abnormal proteins, amyloid-β and tau, in the brain, which is the cause of Alzheimer’s disease. The PET detector ring of this product has an inner diameter of 28 cm, which is smaller than the approximate 80 cm diameter found in a whole-body PET system, so it can take images closer to the target locations. In this way, the resolution of the PET images is increased by a factor of about two compared with a whole-body PET system, so high-definition PET images can be provided.
The United States is ahead of the rest of the world in employing amyloid PET examinations to identify the accumulation of amyloid-β in the brain, for making decisions regarding administration of therapeutic drugs for Alzheimer’s disease, and Shimadzu will respond to this demand for testing with this product. Also, it is expected that the demand for amyloid PET examinations will increase further as the number of cases of Alzheimer’s disease increases in the future and as research progresses. In addition, it is envisaged that the system will be used for imaging the accumulation of the tau protein in the brain, which is another cause of Alzheimer’s disease. The tau protein can accumulate locally in minute areas within the brain, and it is known that there is a correction between these accumulation sites and the symptoms of dementia. With the advancement of research on tau protein-targeted therapies and its distribution in the brain, there is an increasing expectation for the necessity of tau PET imaging using high-resolution PET system.
By continuing to provide this product, Shimadzu will contribute to the early diagnosis and prediction of the progression of dementia, including Alzheimer’s dementia, and to research and development of treatments that aim to delay the onset and progression of dementia.
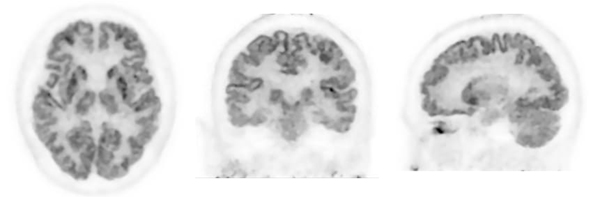
Photos: FDG-PET Images of Brain
(Courtesy of Institute of Advanced Clinical Medicine, Kindai University Hospital)
510(k) Premarket Notification
510(K) Number:K240827
Proprietary Name:PositView, SET-5002
https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRL/rl.cfm?lid=900871&lpcd=KPS
For more details, visit
TOF-PET Scanner for Head & Breast - PositView (SET-5002)
About Shimadzu Medical Systems USA
Shimadzu Corporation, founded in 1875 in Kyoto, Japan and the parent of Shimadzu Precision Instruments, Inc. doing business as Shimadzu Medical Systems USA (SMS), is a global provider of medical diagnostic equipment including conventional, interventional, and digital X-Ray systems. Shimadzu Medical Systems USA is headquartered in Long Beach, CA with Sales and Service offices throughout the United States, the Caribbean and Direct Operations located in Dallas, TX, Kenmore, WA, Vacaville, CA, and the greater Chicago area. Visit SMS at: www.shimadzu-usa.com or call (800) 228-1429.